How do cells spread word upon a viral infection?
For her cum laude PhD research, Laura Van Eyndhoven explored how so-called first responder cells inform immune cells about a viral infection by looking at one cell at a time.
![[Translate to English:] [Translate to English:]](https://assets.w3.tue.nl/w/fileadmin/_processed_/b/3/csm_van%20Eyndhoven%20Immune%20cells%20encapsulated%20in%20microfluidic%20droplets_fd1a150e97.jpg)
Why do some people experience few symptoms, while others become seriously ill, when infected with a virus such as the flu or Covid-19? PhD researcher Laura Van Eyndhoven sought to answer this question by looking at cells one cell at a time. Her research provides valuable insights on how individual immune cells influence systemic immunity, which could lead to personalized therapies for cancer and autoimmune diseases in the future.
Something that puzzles many is the range of symptoms shown by different people when infected by a virus such as Covid-19. Some become very ill, while others display little or no symptoms, despite the fact that they test positive for the virus.
What is the cause of such variations in terms of symptoms? And what role does single-cell behavior and communication play? Well, PhD researcher Laura Van Eyndhoven set out to address these questions and more by looking at how single cells behave when infected with a virus.

The domino effect
“When a virus invades, only a small fraction of the infected cells, so-called first responders, let other cells around them know that they are infected. In other words, the first responders play a special role in letting the body know when it’s under attack,” says Van Eyndhoven, who completed her PhD research in the Immunoengineering group under the supervision of Jurjen Tel and Carlijn Bouten.
To inform uninfected cells and the all-important immune cells that the body is under attack, the first responders produce signaling proteins known as type I interferons (IFN-Is for short). “The production of IFN-Is is a crucial step when it comes to clearing a viral infection,” notes van Eyndhoven. “This is because the cells that first produce IFN-Is kick-start the production of IFN-Is in other cells, including immune cells. It’s sort of like a domino effect.”
Avoid the cytokine storm
Communication using interferons is critical, but communication between immune cells on the production of the right quantity of interferons is also important. “If too much IFN-Is are produced then it increases the chances of someone developing an autoimmune disease, but if too little is produced it could lead to unsuccessful clearance of viruses,” says Van Eyndhoven.
How do things work then with a viral infection like Covid-19 then? Van Eyndhoven explains. “Once infected with Covid-19, it is crucial that cells quickly start making IFN-Is, as this accelerates the process of clearing of the infection. If this process is delayed, and it takes too long for the cells to start producing IFN-Is, the virus can freely replicate, leading to a high viral load, and a catastrophic overshooting of IFN-Is production. This leads to so-called cytokine storms (uncontrolled inflammation resulting in severe life-threatening conditions) and chronic inflammation.”
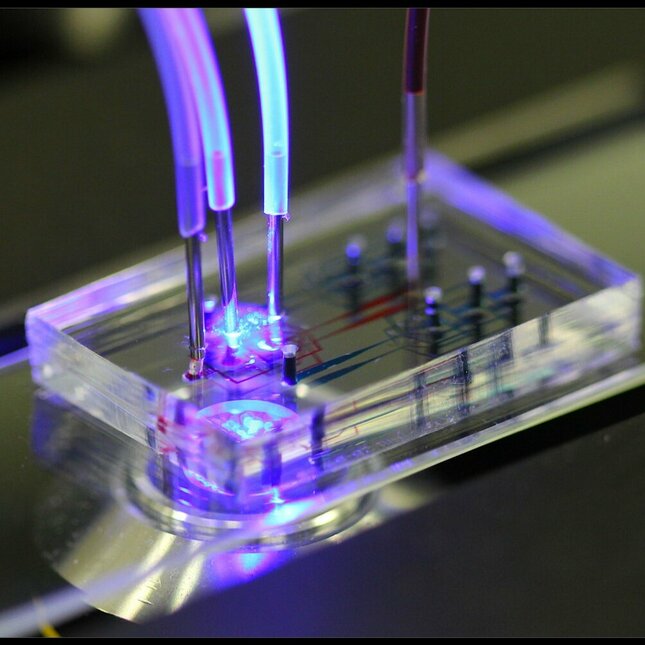
One cell at a time
The varying symptoms exhibited by individuals when infected with viruses, such as the flu or Covid-19, are hypothesized to be attributed to the varying rates of initial interferon production by a small fraction of cells across individuals. “It could be the case that those who develop severe symptoms upon infection have ‘lazy’ first responder cells,” remarks Van Eyndhoven. So, how can this be studied in detail?
“Thanks to recent advances, we now have the technology to study single immune cell behaviors and to manipulate their behaviors. In other words, we can study the cells one cell at a time,” says van Eyndhoven. “And for my research I turned to experiments with primary immune cells, isolated from both healthy donors and patients with autoimmune diseases. In turn, computer models allowed us to interpret the lab results.”
To study single immune cells in the lab, Van Eyndhoven used microfluidic devices. “I was able to study and activate individual immune cells in picoliter droplets, which was virtually impossible in the past. This provided insight into how an individual cell communicates with cells that might be nearby.”
Microfluidic experiments might have helped with understanding individual cell behavior, but it proved very difficult for Van Eyndhoven and her collaborators to study the same behaviors in an in vivo environment. “That’s when we turned to computer modeling as the models can approximate a large cellular system, while at the same time accounting for different things such as interacting molecules, complex tissues, and even the patient.”
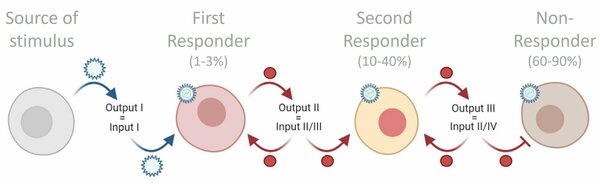
The fate of first responder cells
By combining microfluidics with computer modeling, Van Eyndhoven was able to study interferon production in single cells and compare the results with cases of large groups of cells. She and her colleagues made a startling discovery about the fate of first responder cells that could serve as a major finding when it comes to improving IFN-Is-targeted therapies.
“Only between 1% and 3% of cells are first responder cells - the first cells to produce IFN-Is upon infection. However, first responder cells do not randomly start making IFN-Is upon infection, as was assumed in the past. Instead, my research shows that these cells are destined to be first responders. Their fate is largely pre-determined.”
Future vision
The insights obtained in Van Eyndhoven’s research form the steppingstone towards understanding the fundamentals of how individual cells collectively perform system-level functions and how they can be manipulated for therapeutic treatments.
“I imagine a future where personalized therapies target aberrant cytokine secretion profiles (where single cells make incorrect decisions), as observed in numerous autoimmune diseases,” says Van Eyndhoven.
Further information
Laura van Eyndhoven defended her PhD research cum laude at the department of Biomedical Engineering on March 3rd.
Title of PhD thesis: Decoding type I interferon response dynamics using microfluidics and modeling. Supervisors: Jurjen Tel and Carlijn Bouten.
Media contact
More on Health


Latest news