Tailor-made carbon electrodes for redox flow batteries
In a new collaboration, TU/e and Schunk Group jointly work on scaling up the manufacturing process of novel carbon-based electrodes while simultaneously investigating how to commercialize the product.

Redox flow batteries (RFBs) are stationary, industrial-size batteries that are used to store excess electrical energy produced from renewables, such as solar power or wind energy. Providing access to stored energy when it’s dark or windless will accelerate the transition to sustainable energy. A scientific publication about the non-solvent induced phase separation (NIPS) electrodes that were developed by Antoni Forner-Cuenca and his colleagues drew the attention of Schunk Group, an industrial supplier of carbon components, among other things. In early October, they kicked off their collaboration to research and scale up these NIPS electrodes to a commercial scale at TU/e.
It is not uncommon to find research groups at TU/e collaborating with industry to develop techniques and share knowledge. What sets the collaboration between Schunk Group and Antoni Forner-Cuenca’s Electrochemical Materials and Systems Laboratory apart is not just how they share the responsibilities without compromising the scientific independence of the university. It is the fact that the company believes in this new technology enough to fund the entire project, including a PhD researcher for the fundamental research at TU/e. This will speed up the research and development significantly when compared to the more conventional road of applying for and receiving public funding for the research.
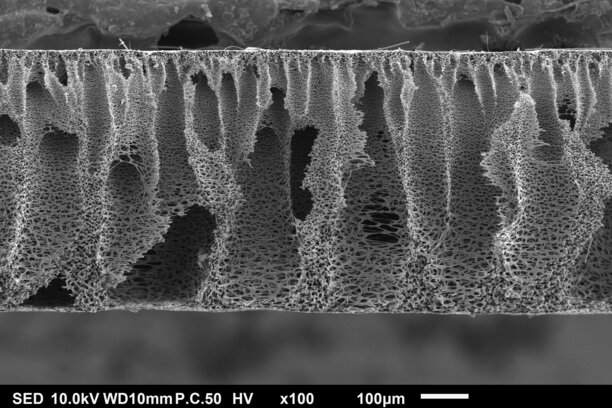
That optimism seems to be entirely justified when considering the technology that this collaboration will concern – a new carbon-based electrode material for redox flow batteries (RFB) which leads to smaller stacks (assembly of serial cells) and lower costs per kW in the entire battery unit. The NIPS electrodes were developed in an international collaboration between TU/e and MIT, as was published in Advanced Materials and Cell Reports Physical Science. PhD students Rémy Jacquemond (supervised by Antoni Forner-Cuenca and Kitty Nijmeijer) and Charles Wan (supervised by Fikile Brushett and Yet Ming Chiang) were the leading researchers in this work.

Inspiring material
“It is an exciting technology to be working on. Doing fundamental research on redox flow batteries helped us to conceptualize what an ideal electrode would look like. We are thrilled that the prepared NIPS materials in the present work have demonstrated promising performance in flow batteries,” explains Forner-Cuenca. “Besides our passion for fundamental research, we like to make a more tangible impact on society and are eager to see our research in the real market.”
“We were intrigued and amazed by the potential of the NIPS electrodes for doubling the power density compared to the best commercial electrodes available today,” adds Hartmut Gross, Director New Business and Technology at Schunk Group. “However, bringing the NIPS electrode to market means scaling two orders of magnitude in size up to 900 cm² in area – there is still scientific research to be done, especially by adopting the chemistry while implementing the process into industrial equipment. Therefore, we need and benefit from each other.”

Co-creation
As it turned out, Schunk Group – already a leading supplier of graphite bipolar plates in the market – was very interested in the NIPS electrodes and believes that they will be instrumental in creating the next generation of redox flow batteries.
Gross clarifies this: “This is why we settled on the approach and collaboration that we have now. The PhD researcher at the university will conduct the fundamental research. At the same time, we have hired a dedicated engineer who will work on reproducibility, robustness, production methods and production design, among other things. In short, he will focus on all the aspects that will make the NIPS electrodes a viable product that we can mass produce. Together, this duo will work closely together and share findings, insights and inspiration both ways.”
The PhD researcher, Simona Buzzi, will carry out the research and the first steps of scale-up, while Hendrik Hemmelmann will contribute with co-development work on Schunk’s side. “We have our partners lining up for implementation testing and reliability tests. We trust we can make it work on an industrial scale in less than three to four years and, in that case, we have faith that our funding will be money well-spent in speeding up a technology that would otherwise not have become available for at least another ten years,” concludes Gross.
Schunk Group
The Schunk Group is a global technology company. The company is a leading supplier of products made of high-tech materials – such as carbon, technical ceramics and sintered metal – as well as machines and systems – from environmental simulation and air conditioning to ultrasonic welding and optical machines. The Schunk Group has around 9,000 employees in 27 countries and achieved sales of €1.3 billion in 2021.
More on sustainability
![[Translate to English:] [Translate to English:]](https://assets.w3.tue.nl/w/fileadmin/_processed_/c/f/csm_BvOF_2024_0319_AEV_license_TUe_Dirk_van_Meer_-_CORE_1__c976e259a5.jpg)


Latest news

![[Translate to English:] [Translate to English:]](https://assets.w3.tue.nl/w/fileadmin/_processed_/e/6/csm_Hendriks%20Banner%20image%20Photonic%20crystal%20fiber-tip%20sensor%20BvOF_9b4093b84b.jpg)