If plaque builds up in the body's arteries, the condition is called atherosclerosis. Over time, plaque hardens and narrows the arteries. This may limit the flow of oxygen-rich blood to organs and other parts of the body. If plaques build up in the carotid arteries and rupture, a stroke can occur.
Currently, intervention planning relies on the severity of the stenosis. However, only one out of six patients benefits from this intervention. So in practice, the severity of the stenosis is not a reliable criterion for the risk of plaque rupture. Plaque rupture occurs when the mechanical stresses in the cap of the plaque exceed the local tissue strength. Therefore, a biomechanical model of the plaque may help to better assess rupture risk. Ultrasound has the ability to provide highly detailed information on plaque geometry and distensibility. Motion tracking techniques can be used to estimate movement of the wall, strains in the wall and flow in the lumen (see ‘Blood in Motion’). However, these data provide quantitative information, and estimates of material properties, but not the actual morphology. A technique that can be combined with traditional ultrasound, in order to provide more information on plaque morphology is photoacoustic imaging.
Photoacoustic (PA) imaging is a novel modality that allows non-invasive imaging of superficial arteries by combining high ultrasound resolution and high optical tissue contrast. In PA imaging, short light pulses are transmitted into the tissue, which are absorbed, generating US signals with its amplitude directly related to its optical absorption. Based on optical absorption contrast of different tissue types, multi-wavelength photo-acoustic imaging can be used to distinguish between different absorbing structures, such as intraplaque hemorrhage and lipid content of the atherosclerotic plaques, which are the most important markers of a plaque prone to rupture (vulnerability of the plaque). Therefore, PA imaging can be used as a better tool to provide a patient specific assessment of the vulnerability of the plaque (the risk of stroke).
In the FULLPHASE project, the world’s first handheld, fully integrated PA probe was developed. Currently an improved PA probe with higher PA SNR is developed in the CVENT project. Combined with the fast laser diode system (1 kHz), a real-time PA imaging of carotid plaque is possible. The pre-clinical and clinical validation is performed at the TUE, focusing on vulnerability detection of atherosclerosis in superficial arteries (this project).
New imaging techniques, experiments and biomechanical models are developed. In this track, the morphology of carotid plaques are characterized using photo-acoustics but also via mechanical characterization using functional ultrasound imaging.
SIGNAL PROCESSING/PHANTOM STUDIES
Although the new PA probe developed in the CVENT project is much improved with a better signal to noise ratio and a reduced size to aid ergonomics, limited laser wavelengths is available in the probe (maximal two wavelengths).
Detecting intraplaque hemorrhages is an important indicator of plaque vulnerability, which is the focus of the CVENT project. Some advanced signal processing makes it possible to detect intraplaque hemorrhages with even a single laser wavelength, 808 nm, which is sensitive to blood. The detection method needs to be able to discriminate between intraplaque hemorrhages that show only wall motion, fast flowing blood in large vessels, but also blood with very low flows in the smaller vessels such as arterioles and the vaso vasorum. Phantoms are created and used to test and validate the performance of the detection method, and first ‘in man’ measurements performed at Catharina Ziekenhuis Eindhoven.
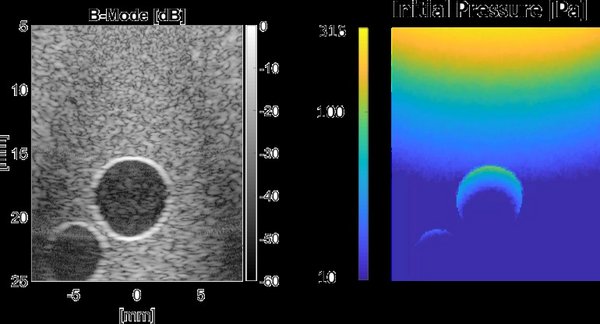
IN SILICO PHOTOACOUSTIC IMAGING TOOLCHAIN
Experiments on phantoms can be valuable to measure the impact of improved acquisitions schemes and signal processing algorithms. However, the fabrication of phantoms can be a difficult and tedious process. Furthermore, phantom studies can suffer from measurement artefacts and the ground truth for (optical) phantom properties is often unknown. Simulations could overcome these issues, because they don’t suffer from measurement artefacts and tissue parameters are known and can be easily tuned. A photoacoustic imaging toolchain is developed that combines Monte-Carlo modelling for the light propagation in tissue, with a numerical method to simulate ultrasound propagation. Furthermore, finite element modelling is coupled to the toolchain to simulate realistic tissue motion due to the beating carotid artery. This toolchain can be used to provide more insight in the behavior of photoacoustics in general and to test new signal processing algorithms in a noise and artefact free environment.
PRECLINICAL VALIDATION
An experimental framework is developed for 3-D PA US imaging with pulsation movement. Before imaging the real human tissue, the PA/US measurements are validated and verified in tissue mimicking phantoms (made of polyvinyl alcohol) of straight tubes, stenotic vessels, the latter even including a fatty plaque, and in biological tissue, i.e., porcine carotids obtained at the local slaughterhouse.
Endarterectomy samples containing the intima and parts of the medial layer of the plaque were obtained from the local hospital (CZE). PA/US imaging on these human samples are performed with pulsation to mimic the in vivo situations. PAUS imaging involves pressurization of the fresh tissue right after surgery, semi-3D PA and ultrasound scanning to obtain geometry, determination of the presence of different constituents, and quantification of wall motion and strain. Micro-CT imaging or histology is performed to validate the morphology assessment.
clinical IN VIVO EXPERIENCE
Initial clinical in vivo PA measurements are currently performed to examine the feasibility of in vivo PA/US imaging of human carotid plaques in the local hospital (CZE) during the endarterectomy operation . Ultimately, a high power multi-wavelength probe will be used for a non-invasive in vivo PA imaging. Although the current imaging setting is only focused on in vivo imaging of hemorrhage.
PROJECTS
Projects for bachelor-end projects, internships and MSc projects are available.
- Development of realistic PA and US phantoms of carotid arteries and plaques including hemorrhage and lipid inclusions
- Combined Echo-CT and multi-wavelength PA-CT imaging of endarterectomy samples
- Noise and clutter reduction algorithm development to improve imaging performance of PA.
- Characterization of the plaque composition using spectroscopic PA imaging based on some spectrum unmixing methods.
Other projects can be designed with the supervisors Roy van Hees (PhD), Jan Willem Muller (PhD), Min Wu (PostDoc) & Richard Lopata.