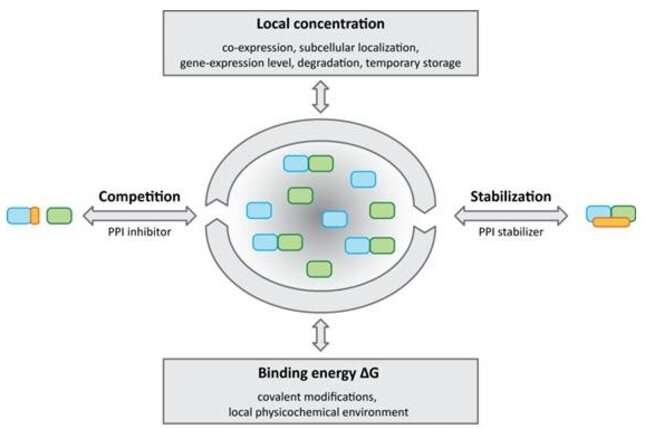
In comparison to the size of the human proteome (~25,000), the estimated numbers of PPIs in the human interactome (650,000) is much larger and therefore offers many opportunities for chemical perturbations of biological systems. Examples for successful small-molecule modulators of PPIs currently under investigation are the Nutlins or ABT-737. Approved drugs that have been found to modulate PPIs are the natural products taxanes and epothilones, which stabilize micro-tubules or the immunosuppressants rapamycin and FK506.
14-3-3 proteins
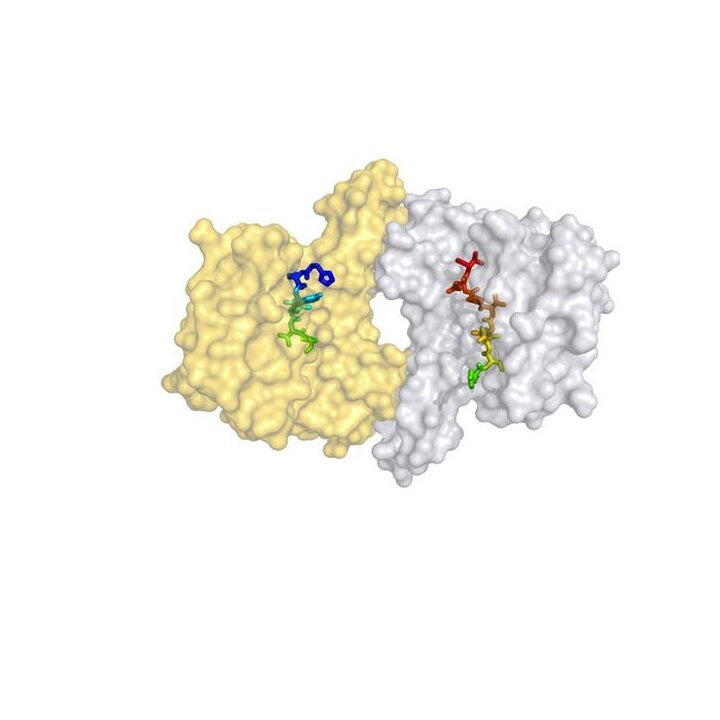
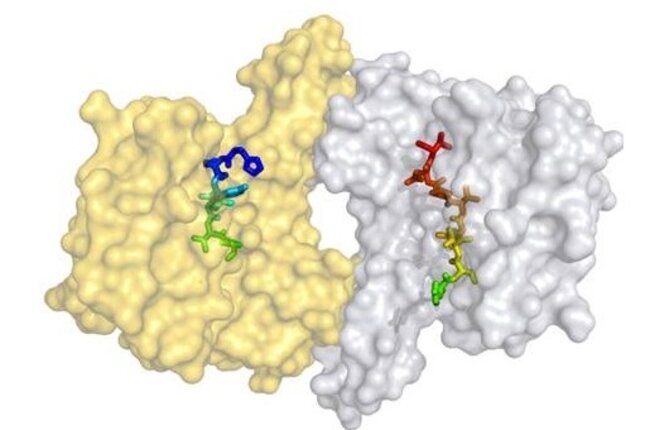
The 14-3-3 adapter proteins comprise a ubiquitous eukaryotic protein family (7 isoforms in humans) that is involved in a wide array of regulatory functions in diverse signalling pathways, cell cycle control and apoptosis. 14-3-3 proteins have no intrinsic enzymatic activity but rather exert their physiological function by directly binding to other proteins. As a consequence, their target proteins are modulated in their enzymatic activity, their subcellular localization or their ability to further bind other protein partners. In this sense 14-3-3 proteins are switchable docking modules controlling the biological activity of their target proteins. To date there are more than 200 protein partners of 14-3-3 proteins known, many of which play prominent roles in the development of diseases like the RAF kinases, p53, Cdc25 phosphatases, FOXO, BAD, YAP/TAZ and TSC1/2. Small-molecule modulation of these interactions by either inhibiting or stabilizing PPIs will produce new tools for the study of cells, tissues and whole organisms.
14-3-3 binding small-molecules
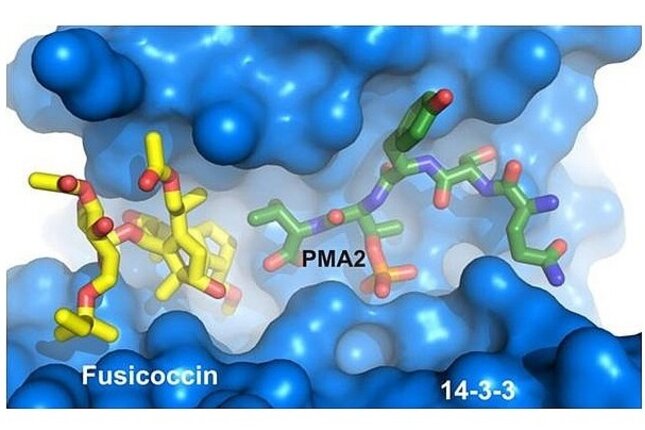
By protein crystallography we have deciphered how the natural product fusicoccin A (FC-A) stabilizes the activated protein complex between the plant plasma membrane H+-ATPase (PMA2) and 14-3-3 adapter proteins. The FC-A molecule binds to the interface of the complex by simultaneously contacting both proteins. We have also discovered chemically less complex molecules in a highthroughput-screening (HTS) that were able to substitute the stabilizing activity of FC-A (epibestatin and pyrrolidone1). Cotylenin A (CN-A), a molecule related to FC-A and showing anti-cancer activity was also crystallized in our group in complex with its protein target. As inhibitors of 14-3-3 PPIs we found a molecule that binds near the phosphate-accepting pocket, and a supramolecular tweezer that binds to lysine near the central binding channel of the 14-3-3 protein. We have furthermore developed a Chemical Induced Dimerisation (CID) system with FC-A as the dimerizing molecule.