Master projects
Master Student Projects - Protein engineering of the CCMV coat protein
Contact
Chiara Pretto (c.pretto@tue.nl), Suzanne Timmermans (s.b.p.e.timmermans@tue.nl), Daan Vervoort (d.f.m.vervoort@tue.nl)
Introduction
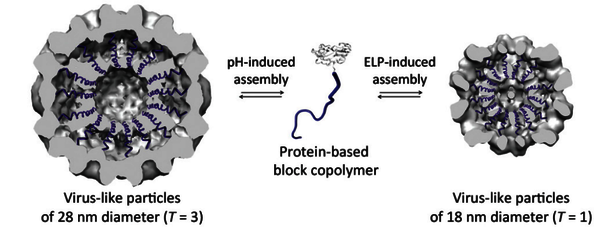
Viral protein nanocages, such as that of the cowpea chlorotic mottle virus (CCMV), are promising carriers for drug delivery applications or capsules for artificial organelle construction. The natural CCMV coat protein assembles spontaneously at pH 5.0 in the absence of its native RNA cargo, while it reversibly disassembles into dimers at pH 7.5. Via introduction of a stimulus-responsive elastin-like polypeptide (ELP) block at the N-terminus of the viral coat protein, we have established capsid assembly under physiological conditions (pH 7.5). As such we are very close to applying the CCMV capsid in vivo.
Summary of the projects and goals
In order to enhance the stability of the ELP-CCMV capsid in vivo, while maintaining its reversible disassembly behavior, the N-terminal ELP block can be modified via protein engineering. Based on a small library of ELP-CCMV variants that are currently available, the master student will design novel ELP-CCMV variants with intermediate hydrophobicity. Via the protein engineering toolbox, these variants can be produced and their assembly behavior and stability should then be evaluated and compared to the currently available variants. If successful, cellular uptake experiments of the assembled capsids can be performed.
Another option for protein engineering of the ELP-CCMV coat protein is to introduce a specific functional handle on the exterior surface of the capsid. This will allow for site-specific modification of the capsid exterior with functional groups that enhance capsid stability or in vivo targeting. In this project, the master student will identify positions on the capsid surface that are suitable for modification based on literature. Protein engineering will then be employed in order to introduce an amino acid with a specific handle, such as a cysteine. Lastly, capsid modification based on the reactive handle will be evaluated.
Techniques
In these projects you will have the opportunity to gain experience with the following techniques (it is not a problem if you don’t have experience with these techniques yet):
- Protein engineering
- Protein expression
- Protein purification
- Analytical methods (i.e. Liquid chromatography-mass spectrometry (LCMS), size-exclusion chromatography (SEC), Dynamic light scattering (DLS), UV-vis spectroscopy, Mass spectrometry, Transmission electron microscopy (TEM))
- Capsid stability analysis
- Labelling via a reactive handle
- (Cellular uptake experiments)
Master Student Projects - Interior and exterior modification of the CCMV capsid
Contact
Chiara Pretto (c.pretto@tue.nl), Suzanne Timmermans (s.b.p.e.timmermans@tue.nl), Daan Vervoort (d.f.m.vervoort@tue.nl)
Introduction
Viral protein nanocages, such as that of the cowpea chlorotic mottle virus (CCMV), are promising carriers for drug delivery applications or capsules for artificial organelle construction. For therapeutic applications a therapeutic agent should be encapsulated inside the capsids, which can be facilitated via modification of the capsid interior. In order to improve the in vivo stability and targeting, the exterior of the capsid can be modified with stabilizing or targeting agents.
Summary of the projects and goals
It has been shown that proteins can be successfully encapsulated via Sortase A-mediated N-terminal modification of CCMV. Hereto an LPXTG-tag (where X is any amino acid) must be present at proteins to encapsulate them via this Sortase reaction. In this project, the master student will develop a method to encapsulate enzymes in which for example, first a click-reaction between an LPXTG-peptide and a therapeutic enzyme is performed, followed by encapsulation of the enzyme via the Sortase reaction. If successful, in vitro studies will be performed to determine the activity of these encapsulated enzymes.
The effect of nucleic acid encapsulation into viral capsids represents a promising field of investigation for several applications such as gene delivery or catalytic activity. In this project the master student will experiment and compare encapsulation efficiency of oligonucleotides and nucleic acids of different lengths and the stabilizing effect of cargo loading. If successful, in vitro toxicity and efficacy studies can be performed.External functionalization of the particle surface could represent an interesting approach in order to increase capsid stability in versatile buffer conditions so expanding the system’s applicability for several purposes. In this project the master student will evaluate different covalent functionalization strategies reported in the literature in terms of applicability to the specific protein cage as well as efficiency and degree of functionalization. A comparison between modification strategies will be followed by the investigation of functionalized particle stability.
In order to control cellular uptake and the location in the body where a therapeutic agent will end up, specific targeting peptides can be used. In this project the master student will design cell-penetrating peptides (CPPs), endosomal escape peptides, and/or specific targeting peptides based on information that is available in literature. Next, these peptides will be synthesized and purified, after which they can be attached to the exterior of CCMV capsids. The degree of functionalization will then be determined as well as the cellular uptake behavior.
TechniquesIn these projects you will have the opportunity to gain experience with the following techniques (it is not a problem if you don’t have experience with these techniques yet):• Peptide synthesis• Protein expression• Protein purification• Chemical modification• Analytical methods (i.e. Liquid chromatography-mass spectrometry (LCMS), size-exclusion chromatography (SEC), Dynamic light scattering (DLS), UV-vis spectroscopy, Mass spectrometry, Transmission electron microscopy (TEM))• (Cellular uptake experiments)
Master Student Project - From amphiphilic copolymers to worm-like micelles and beyond
Requirements:
Master student, Interest in polymer synthesis and nanomedicine
Contact:
Roxane Ridolfo (r.ridolfo@tue.nl)
Introduction
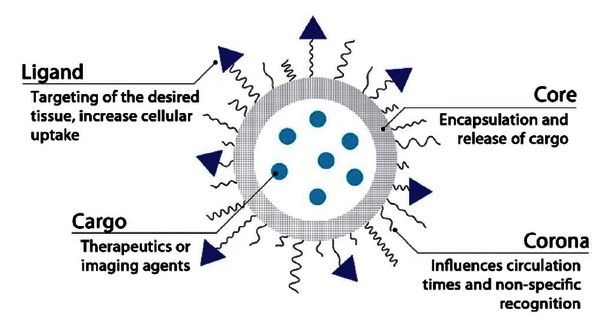
In the past decades, a large number of novel formulations of drug delivery systems have been published. However, only a small percentage of them actually follow their way through clinical trials. One of the parameters of formulation we might forget in order to reach success, is particle morphology. Mimicking design principles and characteristics of natural objects, we can think of engineering functional nanomaterials with different morphologies. Small changes in the chemical components used in any nanoparticle fabrication process (building blocks, solvents, conditions as concentration/temperature/pH/salt behaviour, etc.) can have substantial effects upon the resulting structure (size, shape, stiffness, surface chemistry, etc.), which can obviously change the in vitro/in vivo performance.
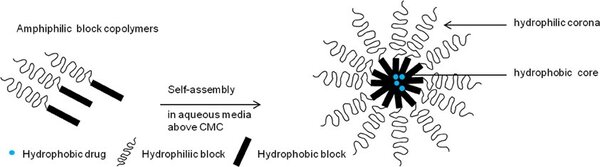
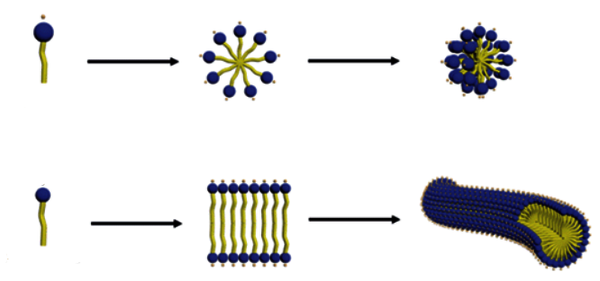
Micelles are self-assembled nanoparticles formed through the hydrophobic re-arrangement of amphiphilic molecules into a thermodynamically favorable spheroid (Fig.1). The hydrophobic portion of the block copolymer will self-associate into the micellar core whereas the hydrophilic portion will form the external corona. Importantly, the micelle core is capable of encapsulating hydrophobic drugs. By varying the right parameters, micelles can undergo a shape change into worm-like structures (Fig.2). This is the aim of the project.
Project goals
Based on the knowledge of our group, the student will synthesize and characterize different biodegradable amphiphilic block copolymers. Polymers will self-assemble into nanoparticles, aiming to produce worm-like micelles. Different characterization techniques (DLS, CryoTEM, fluorescence microscopy…) will be used to evaluate the shape and size of the nanoparticles. This system will further be developed and optimized in order to build a drug delivery vehicle. In vitro cell assays might also be performed.