Reducing global greenhouse gas emissions calls for the development of renewable energy systems. Using solar and wind energy to (photo)electrochemically split water into hydrogen and oxygen presents an interesting approach, where the hydrogen is then used as energy storage medium. Water splitting involves two half reactions: the cathodic hydrogen evolution reaction (HER) and the anodic oxygen evolution reaction (OER). The theoretical minimum potential difference between the two electrodes, required to split water, is 1.23 V. In practice, thermodynamic and kinetic limitations demand significantly higher potential differences, which thus represent an energy loss. The search is for novel electrode materials that reduce this overpotential significantly.
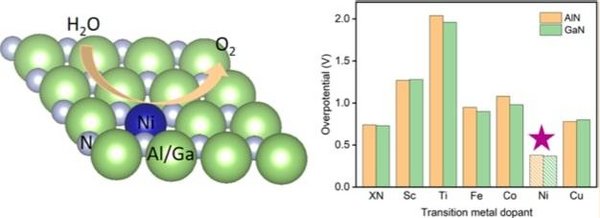
Together with the DIFFER institute we study in our group the fundamental electrochemical reactions of water splitting at the electrodes at an atomistic level, using electronic structure (density functional theory) calculations. The goals are to identify promising materials and reaction centers, and model reaction paths; an example is given in the figure below. There is a strong connection to experimental work on this topic, performed at the DIFFER institute and the TU/e.