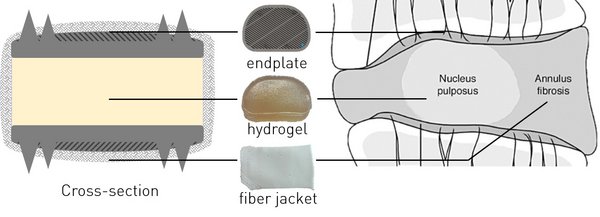
The most commonly performed procedure for treating cervical degenerated disc diseases is anterior cervical discectomy and fusion (ACDF). In this treatment, the dysfunctional intervertebral disc is removed and the two adjacent vertebral bodies are fused together, leading to loss of motion. Cervical artificial disc replacement (C-ADR) has been proposed as an alternative treatment, maintaining physiological cervical mobility. However, clinical results of C-ADR are still not superior to ACDF because problems such as subsidence, facet overloading and migration still occur. Therefore, a new generation of artificial intervertebral discs are under development. Peter van den Broek developed such a prosthesis, which in this thesis is referred to as the bioAID, with the hypothesis that mimicking the native structure of the IVD will lead to mimicking the biomechanical properties of the IVD. The bioAID design contains a hydrogel core, representing the swelling nucleus pulposus, a polymer fiber jacket mimicking the tensile load-bearing annulus fibrosis and metal endplates with pins to connect the prosthesis with the vertebrae as the native endplates do. The first prototypes were developed for the lumbar part, while a prosthesis for the cervical spine showed more commercial feasibility. Therefore, this study focused on a redesigned prototype prosthesis for the cervical spine. To further develop the prosthesis, it is necessary to obtain a good understanding of the mechanical performance of the bioAID.
Therefore, the first aim of this study was redefining the composition of the hydrogel based on its compressive strength, swelling behaviour and ease of manufacturing. These three properties were tested with an unconfined compression test, swelling capacity test and swelling pressure test. The ease of manufacturing was assessed based on the amount of components, production time and reliability of the production process. The tests were executed on 4 different hydrogel designs. All hydrogel designs were able to bear physiological compressive loads without damaging. However, one hydrogel design had a significant higher compressive strength compared to the other hydrogel designs. For all hydrogel designs, a large swelling potential was observed, being high enough to induce a high swelling pressure and pre-stressing of the fiber jacket, such as in a native disc. The most difficult manufacturing was observed for the hydrogel design having the highest compressive strength. Nevertheless, this hydrogel design had the most optimal performance due to the significant higher strength, toughness and swelling pressure. Therefore, this hydrogel composition was used to test the second aim.
The second aim was to evaluate whether the performance of the current design of the complete implant in compression, shear-compression and of the primary fixation method is within the predefined safety requirements. The three parts, being the swelling hydrogel core, the fiber jacket and endplates of the implant, were manually assembled. Next the implants were tested in axial static compression and shear-compression. This study demonstrated that the current design of the implant was able to withstand a static axial compressive load higher than the physiological compressive load. The results of the shear-compression test gave no conclusive value for the ultimate shear strength of the implant, due to limitations of the set-up. For defining the performance of the primary fixation method, a subsidence and device expulsion test was executed. Results of both tests suggested that the current primary fixation method is able to fixate the implant between the vertebral bodies. No evidence of subsidence was found. Moreover, the ultimate expulsion load found in this research was higher than the physiological shear load. The overall results indicate that the current design showed to be mechanically safe in compression and able to remain fixated between the vertebral bodies under physiological loads. Although many steps still need to be performed before this implant can be introduced in the clinic, the results reported here indicate that this design could be a feasible and promising prosthesis for treating severe degenerated disc diseases.