Bone tissue engineering (BTE) is a promising method to regenerate bone by creating three-dimensional (3D) bone tissues via the combination of cells, scaffolds, growth factors and mechanical stimuli. BTE can be utilized for the creation of in vitro bone models. The recreation of artificial bone in vitro, resembling natural bone, has not yet been achieved. As a result, bone tissue processes are not fully understood. In vitro bone models can be used to study and get insight into the behavior of the different cell types in bone tissue. One of the main challenges in the recreation of in vitro bone is the lack of vascularization in 3D bone constructs. Without vascularization, nutrient and metabolite transport must occur entirely via diffusion, which makes reaching the central regions of a 3D scaffold structure very difficult.
The aim of this Master’s project is to establish a proof-of-concept, demonstrating the creation of a vascularized in vitro bone model using cell sheet engineering (CSE) and a silk fibroin (SF) microtube, pre-seeded with endothelial cells. Such an in vitro model would be of interest to study endothelial cell migration and angiogenesis in an environment of bone marrow-derived stem cells (BMSC). CSE is a technology that uses temperature responsive culture dishes to detach cell sheets without the use of proteolytic enzymes. This method keeps the extracellular matrix intact. CSE creates an excellent microenvironment for vascularization since the intact cell matrix is maintained, which is crucial for angiogenesis.
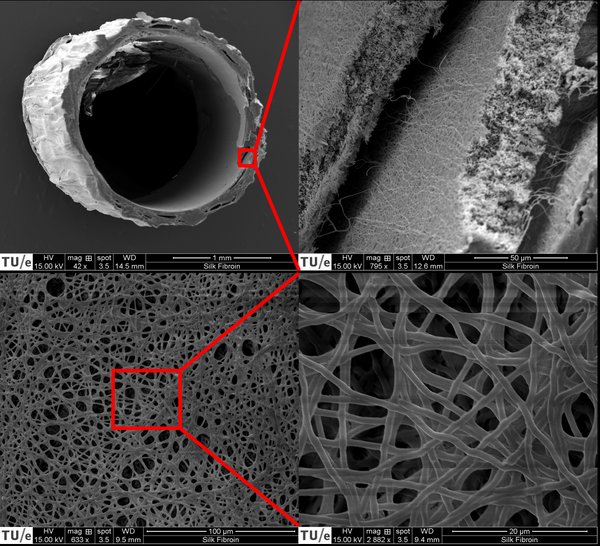
The creation of the 3D construct was performed by combining the technique of CSE with an electrospun SF scaffold. Human umbilical vein endothelial cells (HUVECs) were seeded on the inside of the SF microtube using an in-house manufactured bioreactor. BMSCs were used to create a cell sheet that was wrapped around the outside of the SF tube. The SF microtube was examined with scanning electron microscopy, confocal microscopy was used to evaluate the created 3D constructs on different time points.
Electrospinning of a small diameter SF tube appeared to be a challenging process. Nevertheless, it was accomplished to create an electrospun SF tube that was appropriate for the proof-of-concept of this study. The method to seed HUVECs into the SF tube appeared to be inefficient and is therefore the main limitation of this study. A BMSC sheet with HUVECs seeded on top proved to be functional and the cells remained viable for the duration of the experiment. Further optimization of the cell sheet composition could improve the environment for HUVEC network formation.
Overall, this study has demonstrated the potential of the created 3D construct as a vascularized in vitro bone model. Insight was given into the fundamental principles needed for the creation of such a model. In addition, knowledge was gained about electrospinning of SF into small diameter tubes.