Low back pain is one of the major public health problems in the world, and the burden of low back pain grows each year, due to ageing of the global population. Low back pain is often associated with intervertebral disc (IVD) degeneration. In the healthy IVD, the nucleus pulposus (NP) is contained by the annulus fibrosus (AF) and the endplates of the intervertebral disc. Due to a high concentration of negatively charged glycosaminoglycans (GAGs), healthy NP tissue has a high tonicity. As the NP degenerates, proteoglycans denature and small fragments leach out of the NP tissue, leading to a drop in GAG content in the tissue, and therefore a drop in tonicity. Previous research has shown that NP samples exposed to very low tonicity conditions (free swelling) increased the release of the inflammatory markers prostaglandin E2 and interleukin (IL)-6 into the medium. In a different study, NP tissue was cultured at different tonicities, resulting in an elevated prostaglandin E2 and nitric oxide release into the medium, independent of the change in tonicity of the samples. However, a limitation of this study was the poor reproducibility of the different tonicities. These data suggest that a decrease in tonicity might induce inflammation in the NP tissue, so the aim of the current study was to further investigate the effect of tonicity on NP tissue inflammation.
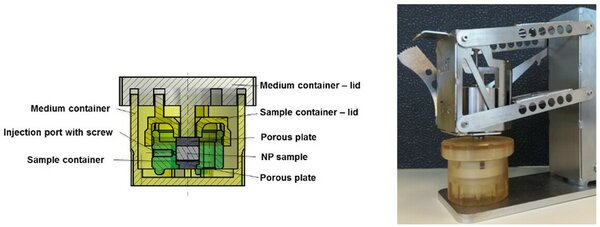
To better control the tonicity of NP tissue, a bioreactor was developed that can be statically loaded. After overnight application of different static loads and locking the sample volume, different tonicities can be induced. To validate the reproducibility of the bioreactor, different osmotic pressures were created by culturing bovine calf NP tissue in the bioreactor under different static loads (0.02 and 0.075 MPa, hypotonic, and 0.2 MPa, native pressure) for 16 hours. Subsequently the volume of the NP was fixed by locking the bioreactor. To measure the osmotic pressure, a stress-relaxation test was performed at 2% strain. The equilibrium stresses of 0.03+/-0.01 MPa, 0.07+/-0.01 MPa and 0.14+/-0.03 MPa respectively demonstrate that three different NP tonicities can be created in a well-controlled manner. However, fixed charge density (FCD) and osmotic pressure of native explants were similar to those of the samples preloaded under the low preloads, indicating the calf NP swelling pressure is different from that of adult bovine and thus human NPs, and that no hypotonic environment was created for the NP cells in this experiment. Moreover, measuring of the calf NP diameter indicated that they were too smal for use in experiments with the bioreactor. Therefore, young bovine NP explants in free swelling and partially constrained swelling conditions were stimulated with an inflammatory stimulus (100 ng/ml tumor necrosis factor (TNF)-alfa and 10 ng/ml IL-1-beta). This resulted in a strong increase in gene expression of IL-1-beta, IL-6 and IL-8 for free swelling NP samples, but a milder response in the partially constrained conditions. The final part of the research aimed to investigate whether a decreased tonicity initiates and/or facilitates an inflammatory response of the NP tissue. In a final experiment, NP tissue was cultured under constrained free swelling conditions within the bioreactor (hypotonic), at 0.02 MPa (hypotonic) and at 0.2 MPa (isotonic), with and without an inflammatory stimulus (100 ng/ml TNF-alfa + 10 ng/ml IL-1-beta) for each tonicity group. The equilibrium stress and the inflammatory response were determined, to evaluate the interplay between hypotonicity and an inflammatory stimulus. Groups with different equilibrium stresses were created, and osmotic pressure and biochemical properties of NP tissue preloaded with 0.2 MPa proved to be similar to native tissue. As gene expression of inflammatory cytokines was not different for hypotonic cultures compared to isotonic cultures after 5 days of culture, it cannot be concluded to what extent hypotonicity influences inflammation in nucleus pulposus tissue.