Description
Cardiovascular diseases (CVD) are the most common causes of death worldwide and new concepts in vascular tissue engineering (TE) and vascular medicine are urgently needed. Each year CVD causes over 4 million deaths in Europe and 1.9 million deaths in the European Union (EU). CVD is responsible for 47% of all deaths in Europe and 40% in the EU. Besides intrinsic factors (e.g. mutations), many CVD pathologies are the result of adverse tissue remodelling. Mechanistic understanding of the remodelling process is required to stop, reverse or prevent pathologies. Vascular tissue remodelling and mechanics are strongly interrelated and remodelling in response to changes in blood flow is important to maintain mechanical homeostasis. Long-term problems linked to vascular remodelling exist in many patient groups with CVD (hypertension with vascular and cardiac hypertrophy, coronary remodelling in atherosclerosis, etc.) and finding ways to prevent these detrimental changes is of high interest. In case of tissue replacement via TE, mechanistic insight is crucial to ensure long-term functionality of engineered tissue. Detailed understanding of the interplay between the mechanical influence of blood flow and vascular remodelling will identify new therapeutic targets and help predict therapeutic outcomes in vascular medicine and engineering.
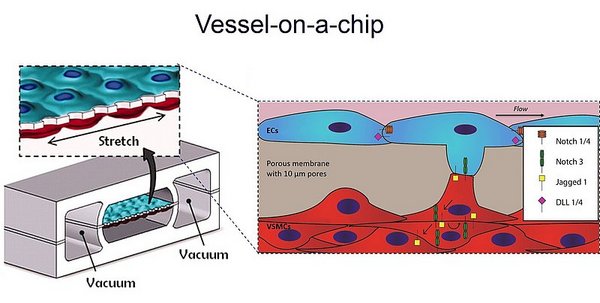
Notch signalling is a key cell to cell signalling pathway in the vasculature which regulates the formation and function of the multi-layered vessel wall. The arterial vessel wall is a multicellular structure with an endothelial cell (EC) sheet surrounded by a layered contractile structure of vascular smooth muscle cells (VSMCs), interlayered with extracellular matrix. ECs, lining the vessel lumen, communicate with VSMCs to regulate the formation and remodelling of wall architecture required to maintain mechanical homeostasis.
The aim of this project is to understand the integration between the mechanics and cell-cell signalling in vascular tissue architecture and homeostasis. The specific focus is on the link between Notch signalling and hemodynamic influence in the formation of the multi-layered vascular wall. It is known that signalling between endothelial cells (ECs) and vascular smooth muscle cells (VSMCs) via the Notch pathway regulates the structure and function of the vessel wall, but how hemodynamic forces integrate with Notch signalling is not understood yet. It has not been possible to answer how these processes interlink using available model systems. By integrating micro tissue engineering, molecular cell biology, and in vivo model systems we will gain mechanistic insight of how cell signalling and hemodynamic forces integrate to control vascular morphogenesis with a specific focus on the arterial wall. Detailed understanding of vascular morphogenesis and physiology will open up new therapeutic possibilities and help us predict therapeutic outcomes in vascular medicine and engineering.
Researchers
Researcher: N.C.A. (Nicole) van Engeland.
Supervisors: C.M. (Cecilia) Sahlgren, C.V.C. (Carlijn) Bouten.
Funding by Åbo Akademi Turku.
Contact
-
Ir. Engeland, N.C.A. van
-
Dr. Sahlgren, C.M.
-
Prof.dr. Bouten, C.V.C.