Maayke Klinkenberg, Tergooiziekenhuizen
Introduction
Tergooiziekenhuizen currently has more than 7500 medical devices, used for the examination, treatment and care of patients. While more and more medical devices are able to generate results digitally, the hospital has not yet developed a policy regarding structural processing, accessing and archiving of this information.
To develop a vision on information management around medical devices and its role within the hospital information architecture, the Cardiology department was chosen as a representative object of study.
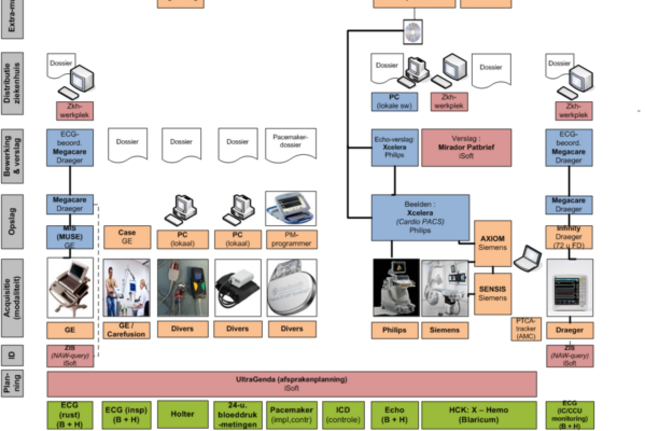
When using a medical device, the following steps are applied: planning, identification, acquisition, storage, processing/reporting, distribution in the hospital and information exchange with external parties. The solutions applied to realize these steps differ per group of medical devices. As a consequence, patient information is scattered among various IT-systems and paper files.
Methods
By studying the present situation through observation, interviews and inventarisations, current generic bottlenecks were identified. The knowledge and insights obtained throughout the curriculum were combined to define a vision and recommendations to eliminate these.
Results
Recommendations to realize this vision have then been implemented in the Cardiology department:
- Application of standardized HL7- and DICOM-connections to implement a fully system-supported workflow for catheterization and ultrasound procedures
- Application of a singular platform for storage of cardiac images
- Distribution of catheterization and ultrasound reports as part of the general hospital information system
- Agreement on the assignment of responsibilities and duties for information and configuration management
In addition, the following recommendations have been formulated to realize the vision hospital-wide:
- Design a hospital information architecture including medical devices
- Determine the generic platforms for storage and disclosure of information generated by medical devices
- Apply automated patient identification when using medical devices
- Select and apply standards for registration and exchange of information
- Assign responsibilities and duties to stakeholders and ensure cooperation

Maayke Klinkenberg PDEng
During her two-year education in Clinical Informatics, Maayke worked in the Clinical Physics and Medical Technology Department of Tergooiziekenhuizen (Hilversum) and was supervised by ir. Guido Zonneveld. Acting as an intermediary between specialists, clinical departments and the ICT-department, she was involved in various acquisition projects for medical devices, focusing on information aspects.