Looking at energy differently provides both obvious and very creative alternatives. We take you through a number of special projects that had their basis here on campus. Some are still in the phase of fundamental research, others are almost in production. And all make an important contribution to reducing our dependence on fossil fuels.
The Heat Battery
This battery is a project of Olaf Aden and a combination of a composite and reactor technology. The basis is a thermochemical principle based on water vapor and a salt hydrate. Once water vapor is admitted to the salt hydrate, the water binds to the salt. This reaction creates a new crystal form and releases heat. As long as you keep the two original components separate, that heat is thereby stored losslessly. And with heat you can reverse that reaction again and start a new cycle.
The battery provides enough heat for e.g. two weeks of hot showering in an average household. Charging can be done with heat and with electricity. So it is ideal for using excess electricity from solar panels or heat from heat pumps.
Hydrogen
When gas was cheap, hydrogen didn't really come into the picture as an alternative. It requires a lot of energy to generate. And that made it an expensive alternative. But the proportions are different now. Hydrogen is becoming interesting again. There is just one hurdle to overcome: the method of making it has not evolved since its invention. And that's because we don't actually know exactly how it works. A team led by Assistant Professor Thijs de Groot is investigating how to take the electrolyzer into the 21e century. You can read about that and why hydrogen as a fuel for cars is not a serious option via the button below:
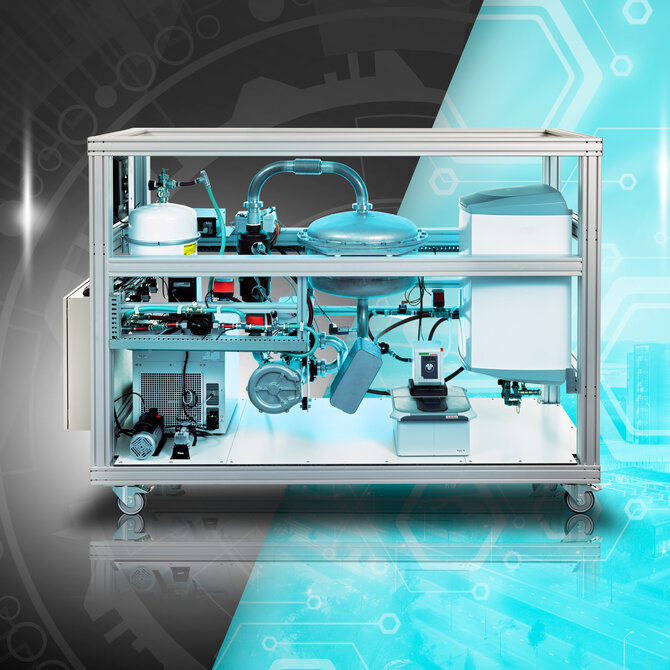
The Redox Flow Battery
While traditional batteries have gotten better and better over the past few decades, they still have serious limitations. Anyone with an electric car notices a decrease in power in winter, they wear out relatively quickly, they require precious and rare raw materials to make, and only if they are properly recycled do they pose no danger to our environment. But even more annoying, they don't have enough storage to play a serious role in the energy transition. Assistant professor Antoni Forner-Cuenca is researching ways to enable scaling up redox flow batteries. And that based on raw materials that are non-polluting and accessible to every economy.
