Upscaling Solvent-Tolerant Nanofiltration: Criteria, Water/Solvent Permeance Analysis, and Synthesis of Epoxy-based membranes
Hakim El Fadil successfully defended his PhD thesis at the Department of Chemical Engineering and Chemistry on March 13th.
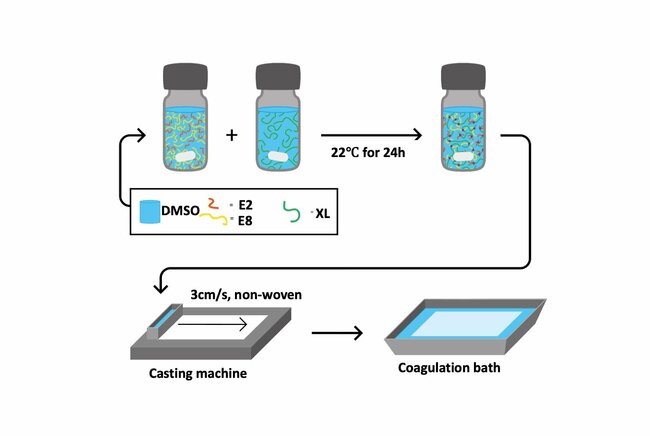
Solvent tolerant nanofiltration (STNF) is vital for cleaning up and recycling industrial water/solvent mixture streams, particularly in the pharmaceutical and chemical production industries. The goal of this PhD was to study how water/solvent mixtures interact with STNF-membranes and to develop highly permeable epoxy-based STNF-membranes transferable to an industrial setting. To achieve this, an advanced testing equipment was built and criteria for transferring new membranes to an industrial setting were established.
An evaluator chart was developed to assess the feasibility of NF-membranes in industry, considering aspects such as performance, cost, ease of manufacturing, toxicity, and waste management. The findings explain the limited transition of nanofiltration membrane chemistries to industry, guiding future research to address techno-economic and environmental considerations for potential industrial adoption.
A high-throughput static filtration (HTSF) apparatus was developed to precisely measure membranes permeance, both in-lab and remotely, especially useful during periods like COVID. The data offers robust insights into NF-membrane and solvent interactions, covering academic testing conditions like constant pressure and industrial settings with constant flux, across varied durations reflective of industrial operations.
Utilizing the HTSF, the study intensively examined the interactions between polymer membranes and solvents. It was found that solvents, such as DMSO, ACN, IPA, or DMF, greatly reduced the permeance of NF-membranes. Increased solvent content within a water/solvent mixture induced changes in viscosity, surface tension, and membrane swelling, all of which contributed to hindering permeation. Furthermore, the passage of water/solvent mixtures triggered clustering of solvent molecules inside the membrane matrix, reducing the permeance, a phenomenon supported by the Zimm-Lundberg theory and NMR analysis.
Given epoxy's unique molecular structure, which aligns well with the stringent demands of solvent tolerance in nanofiltration, it was identified as a prime candidate for membrane development. High-permeance epoxy-based STNF-membranes were then prepared using a non-solvent induced phase separation method, employing a hyper-branched polyethylene imine as the curing agent. These membranes exhibited superior flexibility, permeability, and solvent resistance, suggesting promise for upscaling and purifying solvent-laden water streams.
In conclusion, the research delivers an understanding of STNF-membrane permeance in the presence of specific water/solvent mixtures. Through the precise synthesis of epoxy-based membranes optimized for these mixtures, a milestone in advancing nanofiltration science has been achieved. Future research should harness the extensive knowledge of epoxy chemistries and crosslinking monomers to customize the performance of epoxy-based STNF, addressing specific challenges posed by industrial water/solvent mixture streams.
Title of PhD thesis: Upscaling Solvent-Tolerant Nanofiltration Supervisors: Professor Kitty Nijmeijer