Thermocatalytic Decomposition of Methane in a Gas Fluidized Bed Reactor
Morteza Hadian defended his thesis on October 17th at the department of Chemical Engineering and Chemistry.
Concerns about the destructive effects of global warming and climate change have sparked interest in finding cleaner ways to produce hydrogen, an eco-friendly energy carrier, with low CO2 or no CO2 emissions. One such method is ThermoCatalytic Decomposition of Methane (TCD), which offers advantages over other methods like Carbon Capture and Sequestration (CCS) combined with certain hydrogen production methods. TCD has the potential to create valuable carbon nanomaterials alongside hydrogen, using less energy compared to water electrolysis. This could make it a more attractive and economical choice for industries looking to reduce their environmental impact.

However, using TCD comes with challenges related to the size and weight of catalyst particles. Using certain types of reactors such as a fixed bed reactor could lead to issues like clogging, pressure increase, and reactor damage. A fluidized bed reactor, on the other hand, is better for mixing, heat transfer, and handling larger catalyst particles. It's also easier to use in a continuous manner. Due to the carbon nanomaterial deposition on the catalyst particles design and operation of such reactor is rather difficult and complex. To design a large-scale fluidized bed reactor for TCD, researchers need to understand how catalyst particles grow, how they interact with gas at various temperatures and concentrations, and the reactor's hydrodynamics. This involves experiments in a fluidized bed and complex modeling.
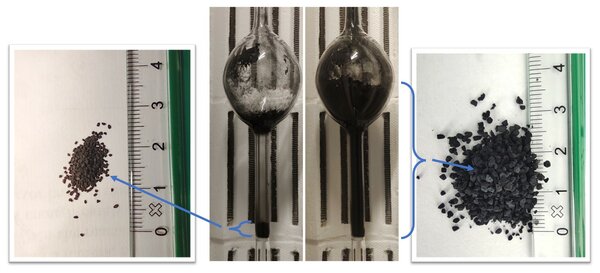
Experiments with TCD in a lab-scale fluidized bed reactor were conducted, exploring the effects of temperature, gas concentration, and space velocity. Valuable carbon nanofibers were produced alongside hydrogen, and their properties were analyzed. The reaction kinetics were described using two parameters: initial reaction rate and deactivation factor.
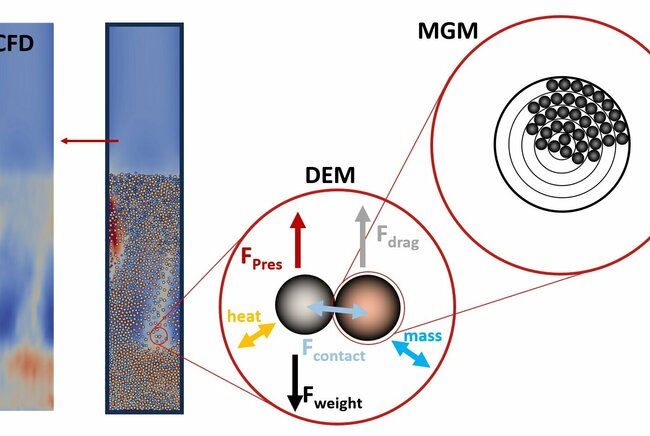
The behavior of catalyst particles over time was modeled using a multi-grain model (MGM). In this model, each catalyst particle consists of solid spherical grains with active material on the surface. The growing solid carbon on the grains' surface impacts particle size, heat and mass transfer and influences the reaction rate.
This MGM model was combined with Computational Fluid Dynamics - Discrete Element Method (CFD-DEM) model, which accounts for hydrodynamic, variations of temperature, and concentration in the gas phase, and movement, collisions and interactions of particles. This combined model on one hand helps understand the impact of fluidization process, mass and heat transfer on the growth rate of catalyst particles over time. On the other hand, it evaluates the effects of growth of catalyst particles on the fluidization regime in the reactor.
The kinetics from experiments were used in the multi-scale model. It's important to manage catalyst particle growth, as they can become too heavy and deactivate. The model was utilized to compare a batch reactor with continuous reactor where to remove larger particles and introduce fresh catalysts in order to maintain performance and viability. These understandings are crucial for the next steps and eventually creating efficient structures and moving closer to industrial-scale application of the process.
Morteza Hadian defended his thesis "Thermocatalytic Decomposition of Methane in a Gas Fluidized Bed Reactor; Numerical Modeling and Experimental Study" on Wednesday 17th of October 2023. He was supervised by Prof.dr.ir. Hans Kuipers and dr.ir. Kay Buist
Latest news


