It’s ‘no sweat’ to use sweat to check your health!
Emma Moonen defended her PhD thesis cum laude, which is about sweat monitoring with a microfluidic device, at the Department of Mechanical Engineering on March 7th as TU/e celebrates International Women’s Day.

We live in a world where smartphones and fitness trackers have made health checking the norm. Heart rate, oxygen saturation, and blood pressure are just some of the things that people follow with devices. To get crucial health information though requires tests on blood samples, which can only be carried out at hospitals and completed by trained personnel. But a blood sample just gives a snapshot of a person’s health at one moment in time. In other words, testing blood samples does not give continuous monitoring. So, PhD researcher Emma Moonen turned to another bodily fluid to solve the problem – sweat. She developed an innovative device that analyzes tiny amounts of sweat from a fingertip or arm and could be used to check for signs of disease or conditions in the future.
Giving a blood sample can be a traumatic process, to say the least. Some people are afraid of needles, some are afraid of the sight of blood, while others are afraid that they’ll faint.
“I’ve got a minor fear of needles, and I don’t like the idea of drawing blood with a needle. So, I’m not a fan of the whole process of going to a hospital to do it,” says Emma Moonen, PhD researcher in the Department of Mechanical Engineering.

The need for blood
Taking a blood sample is often necessary though, particularly when medical personnel need precise information about the body. This information is associated with so-called biomarkers – molecules linked to normal processes in the body, but they could also indicate a condition or disease.
“The drawing of blood is invasive – there is no escaping the needle,” says Moonen. “It also takes time for the medical personnel to take the blood sample, while the patient needs to travel to a specific location, which in some cases is not ideal for them either.”
The use of blood also makes biomarker tracking non-continuous. “Taking a blood sample provides a snapshot of the concentration of biomarkers in the blood at a single point in time. It’s like a photograph of the biomarkers in the blood at one moment in time rather than a movie, which would show how the biomarkers change in concentration over a period.”
Ideally, healthcare professionals and patients alike would both be keen to monitor biomarkers in a non-invasive, continuous, and effortless way. So, to achieve this goal, for her PhD research, Moonen turned to another bodily fluid – sweat!

Precious sweat
“It might sound funny to say this, but sweat is a valuable fluid,” says Moonen. “It’s rich in biomarkers and can be non-invasively collected from the surface of a person’s skin. It’s perfect for continuous monitoring.”
This all sounds too good to be true. But, until now, sweat has been underutilized in clinical settings as a fluid for healthcare monitoring. What exactly is sweat though?
Sweat comes from sweat glands in the skin, with the human body having somewhere between 1.6 and 5 million glands. Mainly made up of water, sweat also contains various other components such as electrolytes (sodium and potassium for example), small amounts of metals (like zinc and copper), as well as glucose, urea (a kidney failure biomarker), and cortisol (a stress biomarker).
“Of course, most people know that the body produces sweat when they exercise, and this is all part of the body’s efforts to cool the body down,” says Moonen. “But the body still produces small amounts of sweat even when a person is at rest. And it’s these tiny amounts of sweat that I wanted to collect and analyze efficiently and accurately.”
Nanoliter challenges
To date, wearable microfluidic devices have been used to collect sweat from people exercising or subject to high temperatures for a prolonged period. “A microfluidic device, which can be made from glass or plastics, contains tiny canal-like channels that can carry miniscule amounts of fluids such as water or – in the case of my research – sweat,” notes Moonen.
But how miniscule do the fluid amounts get? “At rest, a sweat gland might produce 0.1 to 1 nanoliters (nL) of sweat per minute,” says Moonen. To put that in perspective, if a gland were producing 1 nL per minute, it would take almost 2 years for that one sweat gland to fill a liter bottle with sweat!
Fortunately for current sweat-monitoring microfluidic devices, the aim is not to fill a liter bottle with sweat. “Although the amount of sweat needed is much smaller than a liter, it can still take hours, if not days, to fill current microfluidic devices at the low rate of 0.1 to 1 nL per minute per gland.”
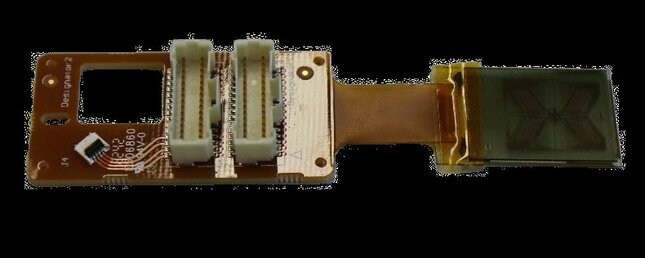
The discretized solution
To overcome the nanoliter challenge, Moonen and her collaborators developed a discretized microfluidic approach to collect small amounts of sweat, and in the process, allow for semicontinuous and noninvasive health monitoring.
“Instead of collecting sweat using one large chamber as in a normal microfluidic device, our device contains several smaller chambers. These chambers are placed on top of the skin, and each can be filled with a few nanoliters of sweat. When some of the chambers are filled, sweat protrudes from the chambers into the device thanks to the pressure from the gland.”

After the sweat arrives, Moonen’s technology breaks the collected sweat up into smaller droplets, with these droplets then transported using electrowetting-on-dielectrics (EWOD). “This changes the wetting properties of a surface using an electric field. Using this, the sweat droplets can be moved in a controlled way to the middle of the device where the sensors are located.”
“We designed a 3D-printed finger-clip to clamp the device to the fingertip. The device can also be attached to the skin using medical tape. This makes it possible to attach the device to different parts of the body, such as a finger or arm, to take measurements from different sites on the body,” says Moonen.
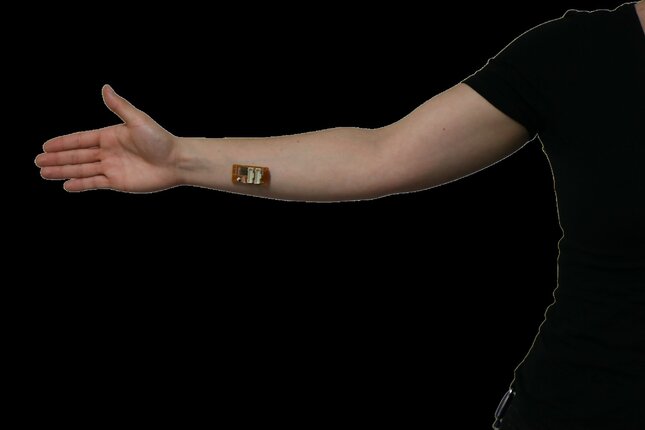
Artificial and real skin tests
To test the efficiency of the device, Moonen performed a series of tests. And the first tests were carried out using artificial skin.
“This is a skin layer made from a silicone material that undergoes a series of treatments to approximate the physical characteristics of human skin. Fake sweat pores are also placed in the artificial skin. The whole artificial skin has the same sweat gland density as a healthy person, and it can deliver the same low sweat rate as a person at rest.”
After testing her microfluidic device on artificial skin, Moonen and her collaborators then tested how well the microfluidic device collects sweat from the skin of several human subjects as a proof-of-principle evaluation of the device.

Goodbye needles?
“We have developed an accurate way to analyze the tiny amounts of sweat produced by someone at rest. This could have a huge impact when it comes to monitoring patient health in hospitals if the technique is developed further.”
For Moonen, the prospect of testing sweat rather than blood in hospitals is a rather attractive one, particularly given her ‘affinity’ for needles and the drawing of blood.
“I’m hoping that my research has a clinical impact sooner rather than later. Because then it would mean that I could have worry less about needles and giving blood samples in the future!”
Title of Emma Moonen's PhD thesis: Discretised microfluidics for noninvasive health monitoring.
Promotors: Jaap den Toonder and Jason Heikenfeld.
Media contact
More on Health


Latest news


![[Translate to English:] [Translate to English:]](https://assets.w3.tue.nl/w/fileadmin/_processed_/e/0/csm_BvOF%202019_1031_BHF%20license%20TUe%20ILI%20copy_8a50884392.jpg)