Vascularization within bone tissue engineered constructs is identified as a major challenge limiting the clinical success. The combination of endothelial cells and bone cells elicits synergistic interactions leading to the development of capillary-like networks within bone tissue. The in vitro vascularization of 3D bone constructs is complex, but is required to mimic the in vivo situation more closely. This study analyzed the abilities of a co-culture consisting of endothelial cells and mesenchymal stem cells (MSCs) within a 3D scaffold, regarding the development of vascular networks and osteogenesis. The performance of two types of stem cells, bone marrow derived mesenchymal stem cells (BMSCs) and adipose derived mesenchymal cells (ASCs), was evaluated in this co-culture system.
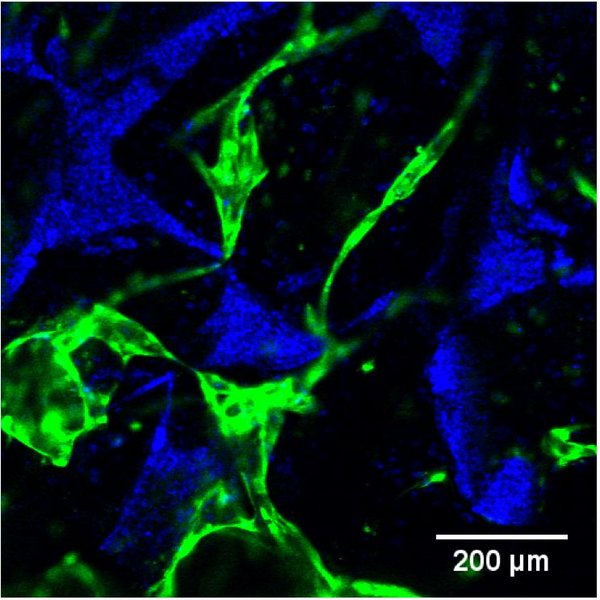
Human umbilical vein endothelial cells (HUVECs) were seeded with BMSCs or ASCs on porous silk fibroin scaffolds. Static culture was performed in endothelial growth medium for 14 days, followed by 14 days in osteogenic medium. Confocal fluorescence microscopy was used to analyze the vascular network formation of GFP-labeled HUVECs. Osteogenesis was evaluated by ALP activity, μCT imaging, calcium content and histological analysis.
Extensively developed vascular networks were observed in both co-culture conditions after 14 days, while a monoculture of HUVECs was not able to arrange in vascular networks. HUVECs co-cultured with BMSCs were able to maintain their vascular networks in osteogenic medium, whereas the vascular network of HUVECs co-cultured with ASCs was disintegrated after 4 days in osteogenic medium. Both co-cultures showed osteogenic activity and mineralization, based on ALP levels, μCT imaging and calcium content. Histological analysis showed higher deposition of bone matrix components in both co-cultures compared to the monocultures ASC and BMSC.
The results indicate a crucial supporting function of both MSC types in the formation of vascular networks, where HUVECs enhance bone matrix formation in both co-culture conditions. These synergistic effects emphasize the importance of the interactions between endothelial and osteoprogenitor cells in the process of bone formation. The ability of this 3D co-culture system to derive vascular networks and induce osteogenesis within silk fibroin constructs during in vitro cultivation may be useful for bone tissue engineering applications, where it may provide a model to assess mechanisms involved in the regulation of vasculogenesis during bone formation or may contribute to personalized treatments.