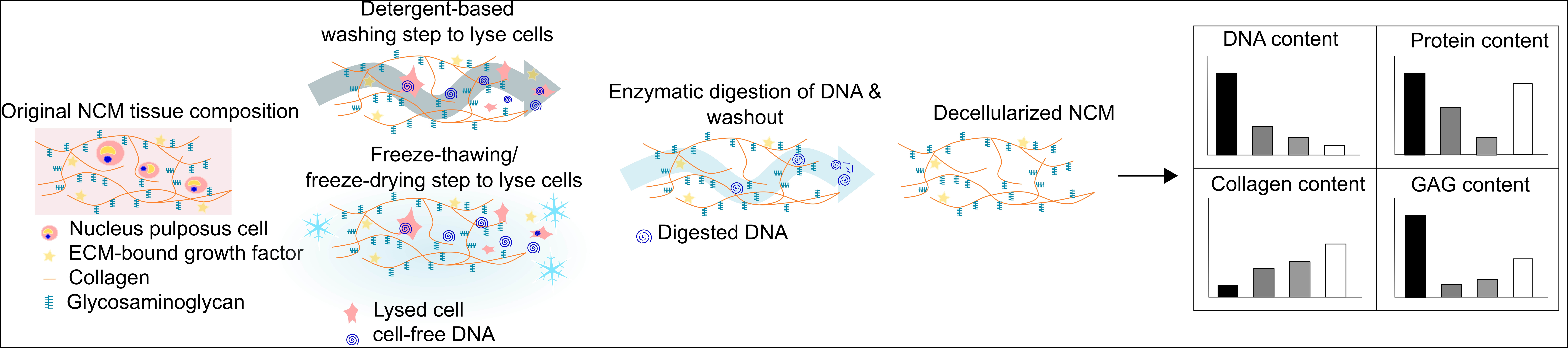
Lower back pain is a common medical problem which can be caused by degeneration of the intervertebral disc (IVD). Current treatment methods focus on pain relief, by for example performing a spinal fusion, or replacing the IVD in late stage degeneration, but do not promote regeneration. Pulverized notochordal cell-rich nucleus pulposus matrix (NCM) enhances matrix production and proliferation of nucleus pulposus (NP) cells and therefore is considered as potential injectable therapy to regenerate the human IVD. However, young pigs are used for the production of NCM. Before considering NCM as a safe treatment in humans, porcine endogenous retroviruses (PERVs) should be removed from NCM via decellularization using endonucleases. The aim of this study is to process NCM to reduce the DNA content while maintaining components considered important for its regenerative capacity in the human IVD, like glycosaminoglycans (GAGs), collagen and proteins.
Porcine NP tissue is decellularized by lysing the cells with detergents or physical methods followed by DNA cleavage with the endonuclease Benzonase and a washout. The biochemical content is analyzed to investigate whether the DNA is reduced to the acceptable level of 50 ng/mg dry tissue weight as recommended by P. Crapo et al. in ‘Biomaterials’ in 2011 and whether GAGs, proteins and collagen are preserved. The remaining Benzonase concentration in the decellularized NP tissue is investigated and tested for cytotoxicity on porcine chondrocytes to ensure biocompatibility.
Use of low Benzonase concentrations resulted in an 80-92% decrease in DNA concentration to 97- 240 ng/mg dry tissue weight when following cell lysis by combined Triton X-100 and deoxycholate or freeze-thaw treatment. Use of high Benzonase concentrations following lyophilization achieved a 91- 92% decrease in DNA concentration to 96-105 ng/mg dry tissue weight. Measured remaining Benzonase concentrations were not cytotoxic to porcine chondrocytes. Both GAG and protein concentration decreased (ranging from a 54-92% and a 13-97% decrease respectively, depending on the decellularization method), while hydroxyproline (as measure for collagen) increased up until 5.65 times the original concentration.
None of the investigated decellularization methods lead to a sufficient reduction of DNA as recommended by Crapo et al. However, the most promising method tested is lyophilization, followed by a high Benzonase concentration and short washout. This loss of components is explained by swelling of NP tissue over time in ultrapure water, showing a weight increase in the first two hours, followed by a weight decrease to 22% (non-lyophilized) and 34% (lyophilized) of the highest measured weight over the period of six days. This indicates that a shorter time in buffer and a smaller buffer volume is more favorable. The use of higher Benzonase concentrations could be investigated to lower the DNA concentration even further in order to process NP tissue for its application as a regenerative biomaterial for the human IVD.