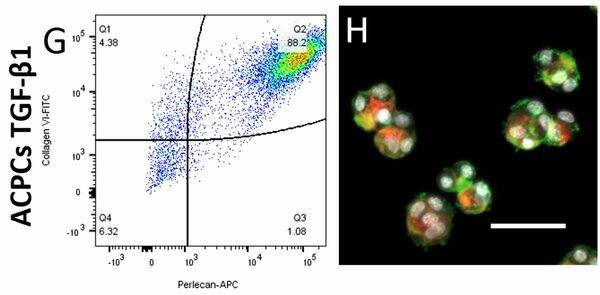
Currently, articular cartilage repair is one of the major challenges in the orthopedic field. One of the more established cell-based therapies, autologous chondrocyte implantation (ACI), too often results in the formation of fibrocartilage by dedifferentiation of chondrocytes and the loss of the pericellular matrix (PCM) during the 2D in vitro expansion. In native articular cartilage, chondrocytes are entirely encapsulated by a PCM, which is together called a chondron. Chondron-based cartilage tissue engineering has shown great potential; however, the current golden standard for isolating chondrons results in a heterogeneous mixture of chondrocytes, partial, and a low yield of intact chondrons. Therefore, an alternative method is needed for obtaining high yields of chondrons. 3D hydrogels have already shown great potential in producing and maintaining PCM formation in chondrocytes. A potential alternative to dedifferentiating chondrocytes is using articular chondroprogenitor cells (ACPCs). ACPCs maintain their chondrogenic phenotype after extensive in vitro expansion, and their chondrogenic potential could be significantly enhanced by adding specific growth factors. Therefore, this study analyzes the potential of creating chondrons using ACPCs, enhanced by different growth factors, inside alginate beads. This study investigated PCM and extracellular matrix (ECM) production by bovine ACPCs (bACPCs), enhanced by bone morphogenic protein 9 (BMP-9), transforming growth factor-beta1 (TGF-β1), or a switch from one-shot BMP-9 to TGF-β1 in alginate beads over a two-week culture period. Biochemical assays, immunohistochemistry, and flow cytometry were used to assess the quantity and quality of the PCM and ECM. The bACPCs, enhanced by growth factors, showed increased formation of PCM & ECM inside the alginate beads, then without growth factors. After seven days, the TGF-β1 and the switch group showed a compact ring of PCM around the cell, similar to the bovine articular chondrocytes, while also showing proliferation at the edge of the bead after two weeks. Still, differences can be found between the two groups. The switch group showed significantly increased proteoglycan production per cell over time, with visible ECM staining, while the TGF-β1 group almost showed no ECM formation. At the center of the bead, less matrix production and no proliferation were seen in these groups. The BMP-9 group showed high diffused production of ECM, with unstructured and diffused production of PCM. Additionally, flow cytometry data showed the preservation of the PCM after dissolving the alginate beads, confirmed by fluorescent microscopy, which makes this culture system suitable for secondary cultures. Altogether, the bACPCs switch group shows to be a promising alternative to chondrocytes for creating chondrons inside alginate beads.