Description
In situ TE (TE) is a new approach for obtaining living replacement grafts for damaged heart valves and blood vessels, superior to existing prostheses. This approach employs acellular, bioresorbable scaffolds that are designed to attract cells and stimulate the formation of new tissue in vivo, directly on the site of destination. During this process, the initial scaffold material is slowly degraded by the cells, resulting in a fully autologous, functional and adaptive replacement tissue. The fundamental principle behind in situ TE lies in the host immune response. Upon implantation, the scaffold triggers a foreign body response, which in turn activates a wound healing cascade leading to healthy regeneration or repair (scar formation). In case of the heart valve, this process occurs in the stringent hemodynamic environment, imposing local shear stresses and cyclic strains on the valvular scaffold. Our philosophy is that it is possible to modulate the scaffold-induced immune response via the physical properties of the scaffold (e.g. microstructure, mechanical properties). Therefore, it is essential to learn how the immune cells respond to biophysical and biomechanical cues.
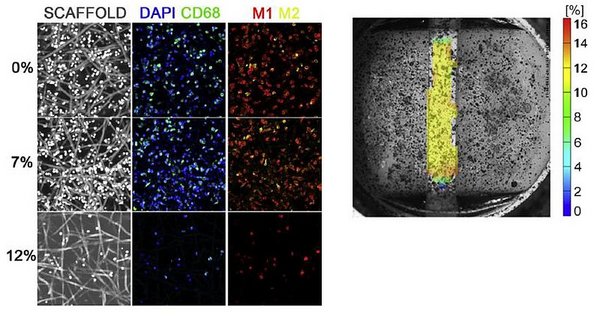
Macrophages are believed to be the key mediators in the wound healing cascade. In fact, it was shown that ablation of macrophages leads to a complete lack of healing or regeneration. Macrophages are very plastic cells that can exert an array of functions by polarizing towards different phenotypical states, with on the extremes the pro-inflammatory ‘M1’ state versus the pro-wound healing ‘M2’ state. This is a key feature for the in situ TE application as the initial macrophage response towards a biomaterial is proposed to be predictive for long-term regeneration. Recent data suggests that macrophage polarization can be tuned via the scaffold microstructure (e.g. fiber diameter) and previous results in our group revealed that the polarization state of macrophages in a 3D scaffold is affected by cyclic strain (Fig. 1). However, a mechanistic understanding of the macrophage response to these combined stimuli is lacking. Therefore, the goal of this MSc project is to elucidate the response of human macrophages to cyclic strain in 3D electrospun scaffolds with varying microstructures, in terms of macrophage polarization state and potential transdifferentiation towards myofibroblast-like cells. The results of this research will be translated to in situ TE heart valves and potentially to improve the local and possibly anisotropic mechanical properties of next-generation valvular scaffolds.
Supervisors: Anthal Smits & Carlijn Bouten.
Contact
-
Dr.ir. Smits, A.I.P.M.
-
Prof.dr. Bouten, C.V.C.